Identification and Validation of a CCEP-Derived Computational Marker of the Epileptogenic Zone
Team: Team Ragdoll
- Program: Biomedical Engineering
- Course: Precision Care Medicine
Project Description:
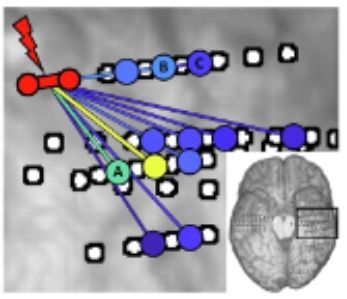
Cortico-Cortical Evoked Potentials (CCEPs) have been used to map regions of brains, but they have never been used for Epileptogenic Zone (EZ) localization. We show that CCEP-based modeling (Random Forest model) can be a promising tool to aid passive EEG / MRI EZ localization methods.
Project Poster
Open full size poster in new tab (PDF)
Project Post Summary:
Epilepsy affects 50 million people globally. 30-40% of patients with epilepsy have medically refractory epilepsy (MRE), which cannot be completely controlled by currently existing drugs and whose treatment accounts for 80% of the $16 billion spent on treating epilepsy annually. For these patients, first-line treatment is the surgical removal of the epileptogenic zone (EZ), but this requires accurate identification of the EZ. This remains a challenge, with surgery success rates ranging from 30-70%. The current gold standard for identifying EZ is to passively capture and analyze a plethora of neurophysiology data, a largely manual process that can take over two weeks.
We are engineering an innovative machine-learning tool trained on cortico-cortical evoked potential (CCEP) data actively measured from single pulse electrical stimulation. This project aims to identify and validate a computationally-derived CCEP-based marker to accurately identify areas of the brain likely to contain the EZ, suggest possible EZ regions that were not identified by conventional analysis, and predict successful or unsuccessful outcome after resection surgery.
To develop our CCEP computational marker, we have trained a random forest model on a combination of CCEP stimulation, response, and network-extracted features from 19 MRE patients that had successful surgical outcomes. Our model performs at a sensitivity of 92% and specificity of 75%. It can identify the EZ agnostically across brain regions as well. We envision this model integrating into the clinical workflow for MRE surgery. Clinicians can leverage our CCEP marker as a decision-support tool to increase EZ localization accuracy and improve surgical outcomes. Future work involves model evaluation within the clinical workflow, with some preliminary results on patient cases shown today.
Student Team Members
- Shreya Singh
- Dongwoo Lee
- Samantha Tam
- Lelin Zhong
- Zaiwei Liu
- Cindy Wang