Driving Gene Delivery from Pipette to Patient
Team: Advanced BME Design Team: HydroGene
Program:
Biomedical Engineering
Project Description:
Worldwide, over 4M patients above the age of 25 are affected by liver-based monogenic hereditary disorders. Wilson’s Disease, affecting roughly 9,000 patients in the United States, is a burdensome liver-based genetic condition. The current standard of care for these patients is a combination of copper chelators and zinc salts to manage symptoms, resulting in annual medical costs over $300K. Additional hospitalizations can further result in costs upwards of $1M. Gene therapy has been proposed as a potential cure for these costly and difficult-to-manage conditions, as it can replace a missing or defective gene and treat the disease. Traditionally, gene therapy is delivered through the bloodstream via adeno-associated viral vectors (AAVs). However, AAVs can trigger an immune response, as many patients have preexisting antibodies that fight AAVs. Additionally, due to its small genome, AAVs require complex and expensive bulk manufacturing systems. An alternative to viral delivery, liver-directed non-viral gene delivery can be accomplished through hydrodynamic injection of DNA to transfect hepatocytes. HydroGene’s injection device integrates into a common endoscopy procedure, ERCP, that accesses the liver via the biliary tree, and ensures patient safety throughout the procedure. The gene delivery approach is versatile and applicable to an array of monogenic disorders and compatible with a wide range of therapeutics, including DNA, RNA, and protein-based medicines. With a safer, more cost-effective method of gene delivery, HydroGene aims to increase accessibility of gene therapy to patients with otherwise incurable genetic conditions.
Team Members
-
[foreach 357]
[if 397 not_equal=””][/if 397][395]
[/foreach 357]
Project Mentors, Sponsors, and Partners
Clinical Mentor: Dr. Vivek Kumbhari, M.D., Ph.D.
Clinical Mentor: Dr. Robert Kruse, M.D., Ph.D.
Faculty Mentor: Shababa Matin
Dr. Yuting Huang, M.D., Ph.D.
Thomas McGuire, M.S.
Dr. Florin Selaru, M.D.
Dr. Jordan Green, Ph.D.
Tom Benassi
Teaching Assistant: Jordan Shuff
Course Faculty
-
[foreach 429]
[if 433 not_equal=””][/if 433][431]
[/foreach 429]
Project Links
Additional Project Information
Project Photo
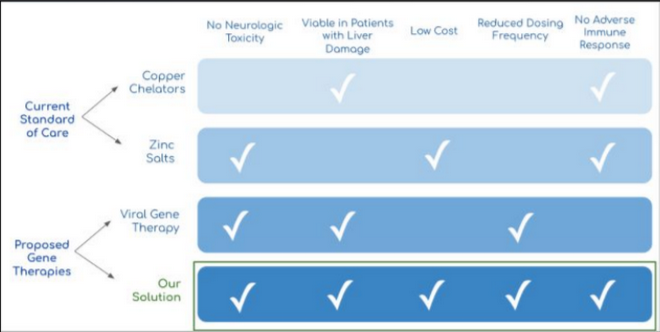
Project Photo Caption:
Wilson’s disease patients are commonly treated palliatively with copper chelators and zinc salts. HydroGene offers a lower cost therapy with an increased safety profile and reduced dosing frequency.


