Analyzing Mutant FGFR3 Association Using Fluorescence Fluctuation Techniques
Program:
Materials Science and Engineering
Project Description:
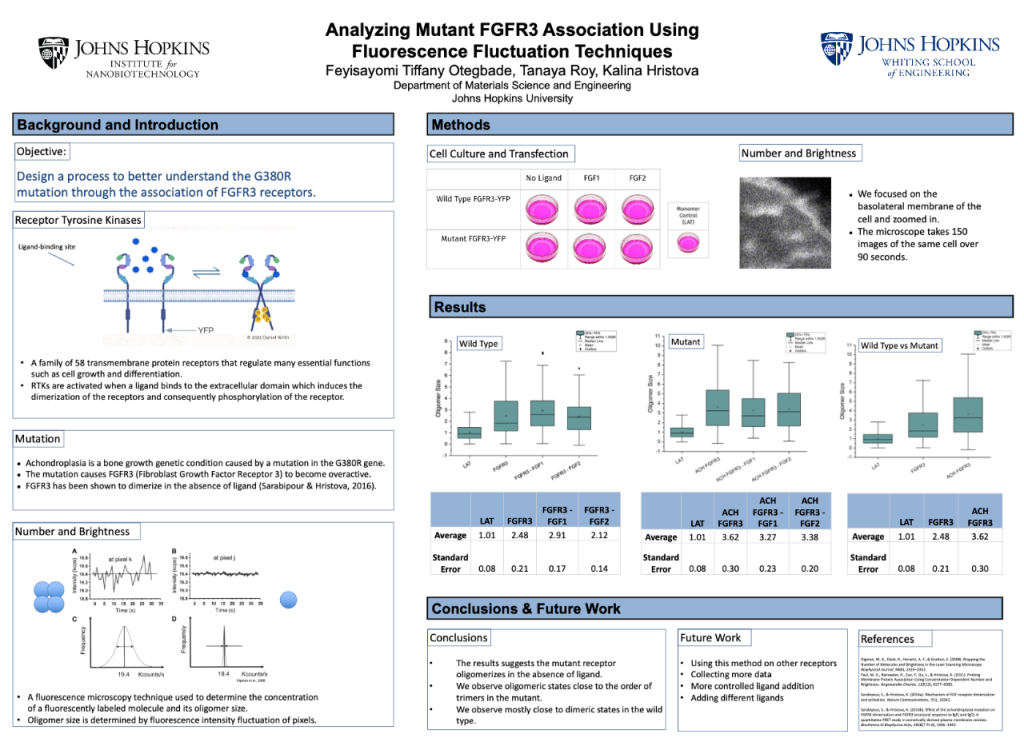
Fibroblast growth factor receptor 3 (FGFR3) is a part of the receptor tyrosine kinase (RTK) family: a family of 58 transmembrane protein receptors that exhibit enzymatic activity. RTKs regulate many essential functions such as cell growth and differentiation. They have similar structures: a ligand-binding site in the extracellular domain, a single transmembrane helix, and a cytoplasmic region containing the protein receptor kinase (Lemmon et. al., 2010).
If the RTKs are functioning properly, they activate when a ligand (a binding molecule) binds to the extracellular domain site. The binding of these growth receptors induces the dimerization of the receptors and consequently phosphorylation of the receptor.
The dysfunction of these receptors can lead to cancer and genetic conditions. Achondroplasia or dwarfism is due to the G380R genetic mutation of FGFR3. Mutant FGFR3 becomes overactive and halts necessary bone growth. In this case, researchers have found that the G380R mutation increased FGFR3 dimerization in the absence of ligand (Sarabipour & Hristova, 2016). The methods used previously can only measure up to a dimer configuration. Because these receptors prove to be active and dimerize, even in the absence of ligand, knowledge about higher-order oligomerization can reveal further activity.
In this project, we design a process to better understand this mutation. We used Number and Brightness (N&B) analysis to determine the oligomer size of the mutant in the presence and absence of ligand. Knowledge of higher-order oligomerization will give more insight into the mutation. The oligomerization size of the domains is important because they correspond to receptor activity. Furthermore, this experimental process can be used to analyze mutations in other RTKs and can inform therapies to combat these pathologies.
Team Members
-
[foreach 357]
-
[if 397 not_equal=””][/if 397][395]
[/foreach 357]
Project Mentors, Sponsors, and Partners
-
Tanaya Roy
-
Kalina Hristova
Course Faculty
-
[foreach 429]
-
[if 433 not_equal=””][/if 433][431]
[/foreach 429]